
Use INBRIJA as needed* to fight Parkinson's
symptoms when they return.
*1 dose (2 capsules) per OFF Episode,
no more than 5 doses per day.
INBRIJA is not a substitute for your regular carbidopa/levodopa medication.
Jimmy uses INBRIJA WHERE he wants to use it...
I’ve been using it a couple of years now and I’ll do it in front of anybody, in a restaurant or anywhere.
—Jimmy, person with Parkinson's
Actual Patient. Individual Results may vary.
How to Use INBRIJA
Needing a few tries to get used to using your inhaler is normal when getting started with INBRIJA.
Don't feel discouraged—that's normal! Peter is here to help with a step-by-step guide on how to use INBRIJA.
If you have more questions, check out more of Peter's videos for extra guidance about INBRIJA.
Have more questions?
Peter is here to help. Check out other Peter videos, like "When to Use INBRIJA" and "What Can I Do to Help With Cough When Using INBRIJA?"
More Just Ask PeterSM VideosNurse Educators are here to help

Actor portrayal.
Nurse Educators are available to support you with additional training by phone or video and to answer any questions about how to use the inhaler.
You may contact them at1-833-INBRIJA8 AM to 8 PM ET.
Monday through Friday
Monday through Friday
You may contact them at1-833-INBRIJA8 AM to 8 PM ET.
Monday through Friday
Monday through Friday

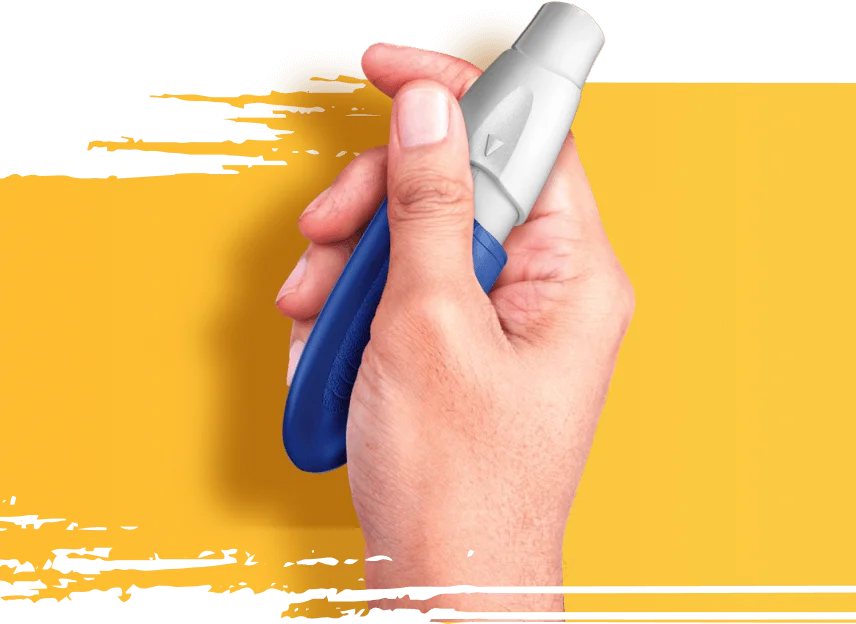
Use INBRIJA as needed when symptoms return in between doses of your carbidopa/levodopa medicine.
- Do not orally inhale more than 1 dose (2 capsules) for any OFF period. Do not take more than 5 doses (10 capsules) in a day
- Do not stop taking your daily Parkinson's medicine. INBRIJA does not replace your regular carbidopa/levodopa medicine
- Do not swallow or open INBRIJA capsules. Only use INBRIJA capsules with the INBRIJA inhaler
- Do not use the INBRIJA inhaler for any other medicine.
Using INBRIJA may take some practice at
first. Below are some helpful hints.
 Stand or sit up straight and hold the inhaler level and away from your mouth. Breathe out completely. Do not breathe into the mouthpiece.
Stand or sit up straight and hold the inhaler level and away from your mouth. Breathe out completely. Do not breathe into the mouthpiece. Close your lips firmly around the mouthpiece. Breathe in slowly and gently, enough to hear or feel the capsule whirl (this means you are getting your medicine).
Close your lips firmly around the mouthpiece. Breathe in slowly and gently, enough to hear or feel the capsule whirl (this means you are getting your medicine). You may take more than one breath per capsule if it's easier for you
You may take more than one breath per capsule if it's easier for you It is quite common to cough as you breathe in, but try not to. If you cough out the medicine, it will reduce the amount you receive in your body.
It is quite common to cough as you breathe in, but try not to. If you cough out the medicine, it will reduce the amount you receive in your body.- – Some people have found that sipping liquid before and after breathing in the medicine helps with cough
- – As you're breathing in, if you feel like you might cough:
- Hold your breath for a few seconds
- Wait until the feeling goes away
- Take a sip of liquid
- Breathe in again with the same capsule
- – For some people, coughing may feel like the sensation of choking
- – And don't forget, if you cough, it's expected. Just breathe in again with the same capsule
 To clean the inhaler, use a new, dry cotton swab to wipe the powder off of the holes from both sides of the mouthpiece with a circular motion. (See Step 14 in Instructions For Use).
To clean the inhaler, use a new, dry cotton swab to wipe the powder off of the holes from both sides of the mouthpiece with a circular motion. (See Step 14 in Instructions For Use).- – You can also use a dry tissue to wipe the outside of the mouthpiece
- – Do not clean any other parts of the inhaler. Do not rinse the mouthpiece or get the inhaler wet

INBRIJA® (levodopa inhalation powder) IMPORTANT CONSUMER SAFETY INFORMATION
What is INBRIJA?
INBRIJA is an inhaled levodopa prescription medicine used to treat the return of Parkinson’s symptoms (known as OFF episodes) in people with Parkinson’s disease who are treated with carbidopa/levodopa medicines. INBRIJA does not replace regular carbidopa/levodopa medicine. It is not known if INBRIJA is safe or effective in children.
Do not use INBRIJA if you: take or have taken a nonselective monoamine oxidase inhibitor such as phenelzine or tranylcypromine within the last 2 weeks.
Before using INBRIJA, tell your healthcare provider about your medical conditions, including if you:
- have asthma, chronic obstructive pulmonary disease (COPD), or any chronic lung disease
- have daytime sleepiness, sleep disorders, sleepiness/drowsiness without warning, or use medicine that increases sleepiness, including antidepressants or antipsychotics
- have dizziness, nausea, sweating, or fainting when standing up
- have abnormal movement (dyskinesia)
- have mental health problems such as hallucinations or psychosis
- have uncontrollable urges like gambling, sexual urges, spending money, or binge eating
- have glaucoma
- are pregnant or plan to become pregnant. It is unknown if INBRIJA can harm your unborn baby.
- are breastfeeding or plan to breastfeed. Levodopa can pass into breastmilk and it is unknown if it can harm the baby.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
Using INBRIJA and certain other medicines may affect each other and cause serious side effects. Especially tell your healthcare provider if you take:
- MAO-B inhibitors
- dopamine (D2) antagonists (including phenothiazines, butyrophenones, risperidone, metoclopramide)
- isoniazid
- iron salts or multivitamins that contain iron salts
How should I use INBRIJA?
INBRIJA is for oral inhalation use only. Do not swallow or open INBRIJA capsules. Only use INBRIJA capsules with the INBRIJA inhaler. Do not use the INBRIJA inhaler to take any other medicine.
Do not orally inhale more than 1 dose (2 capsules) for any OFF period. Do not take more than 5 doses (10 capsules) in a day.
What should I avoid while taking INBRIJA?
Do not drive, operate machinery, or do other activities until you know how INBRIJA affects you. Sleepiness and falling asleep suddenly can happen as late as a year after treatment is started.
What are the possible Side Effects of INBRIJA?
INBRIJA can cause serious side effects including:
- falling asleep during normal daily activities with or without warning. If you become drowsy, do not drive or do activities where you need to be alert for your safety or the safety of others. Chances of falling asleep during normal activities increases if you take medicine that cause drowsiness.
- withdrawal-emergent hyperpyrexia and confusion (fever, stiff muscles, or changes in breathing and heartbeat) if you suddenly stop using INBRIJA or carbidopa/levodopa, or suddenly lower your dose of carbidopa/levodopa.
- low blood pressure when standing up (that may or may not happen with dizziness, fainting, nausea, and sweating). Get up slowly after sitting/lying down.
- hallucinations and other psychosis - INBRIJA may cause or worsen seeing/hearing/believing things that are not real; confusion, disorientation, or disorganized thinking; trouble sleeping; dreaming a lot; being overly suspicious or feeling people want to harm you; acting aggressive; and feeling agitated/restless.
- unusual uncontrollable urges such as gambling, binge eating, shopping, and sexual urges has occurred in some people using medicine like INBRIJA.
- uncontrolled, sudden body movements (dyskinesia) may be caused or worsened by INBRIJA. INBRIJA may need to be stopped or other Parkinson's medicines may need to be changed.
- bronchospasm - people with asthma, COPD, or other lung diseases may wheeze or have difficulty breathing after inhaling INBRIJA. If this occurs, stop taking INBRIJA and seek immediate medical attention.
- increased eye pressure in patients with glaucoma. Your healthcare provider should monitor this.
- changes in certain lab values including liver tests.
The most common side effects of INBRIJA are cough, upper respiratory tract infection, nausea, and change in the color of saliva or spit. Another side effect of INBRIJA is sensation of choking right after use.
These are not all the possible side effects of INBRIJA
- Call your doctor for medical advice about side effects.
- You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
The risk information provided here is not comprehensive. To learn more:
- Talk to your health care provider or pharmacist.
- Visit www.inbrija.com to obtain the Full Prescribing Information, Patient Information, and Instructions for Use.
- Call 1-800-367-5109
INBRIJA® Indication
Treats OFF periods in adults taking carbidopa/levodopa (CD/LD). INBRIJA doesn't replace CD/LD.
Important Safety Information
Don't use if you have taken a nonselective monoamine oxidase inhibitor (eg, phenelzine, tranylcypromine) within the last 2 weeks.
SEE MORE 






